Landline:+86-0775-84649797 Phone:+86 19926651112(Same WeChat ID)
- Home >
- Products >
- RFID Industry label >
- Medical Device Tags

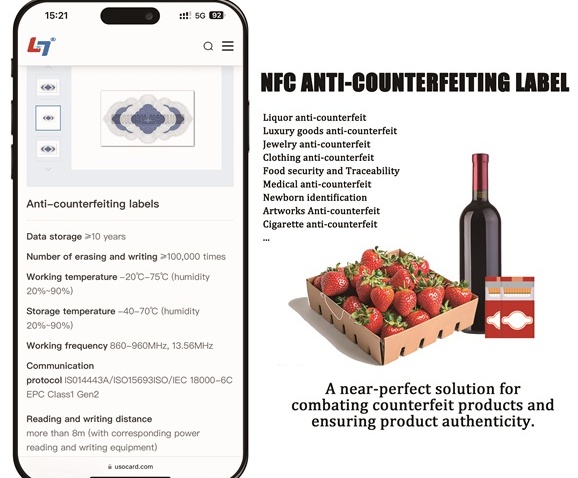
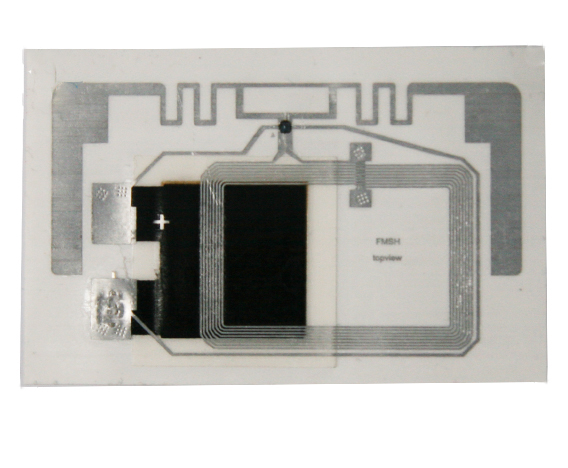
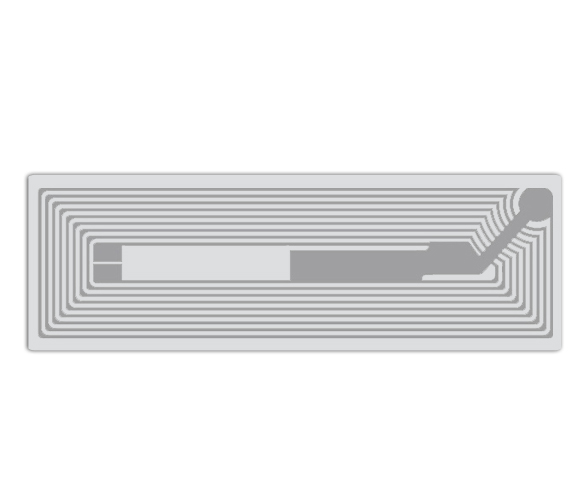
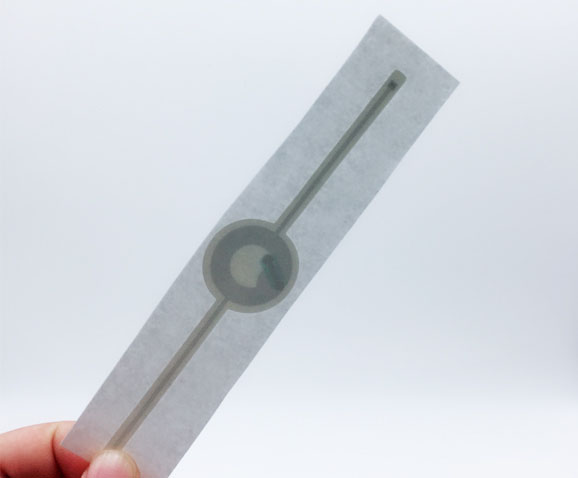
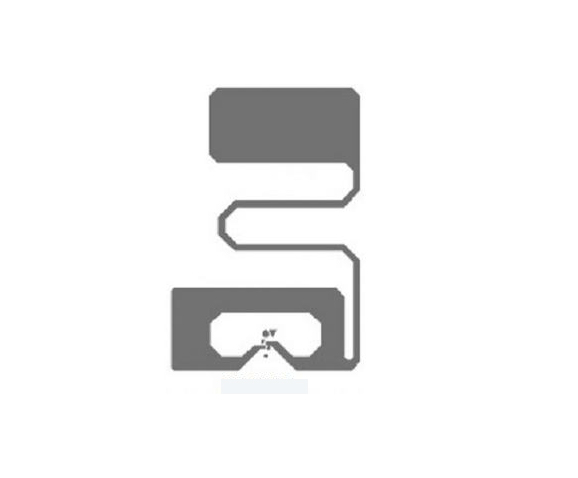
Medical Device Tags
Medical Device Tags refer to signs that are pasted, fixed, and hung on medical devices to indicate product information. Medical device labels should comply with regulatory requirements to ensure the safety and efficacy of medical devices
Inquiries
Product information
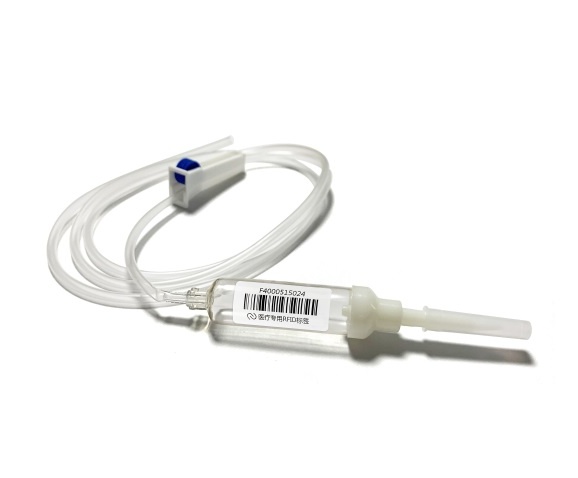
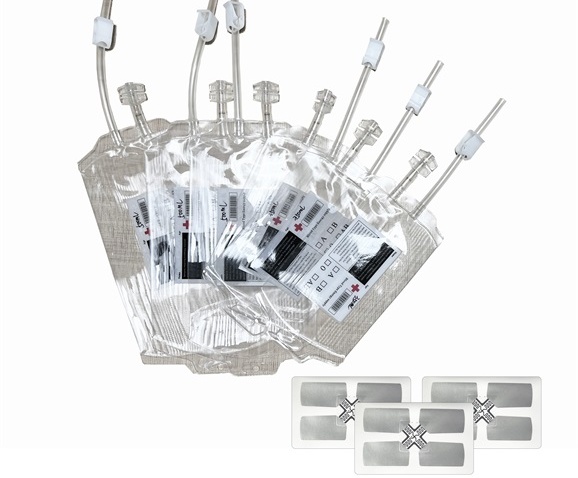
Medical Device Tags
Medical device labels should contain the following parameters:
1. Product name and model
2. Batch number and production date
3. Use and indications
4. Method of use and precautions
5. Storage conditions and expiration date
6. Safety performance parameters
7. CE certification and other certifications
8. Approval number or registration number
Applications
1. Medical equipment labels: Medical equipment labels are usually used to identify equipment parameters such as power and voltage, as well as to print barcodes, product models, and manufacturer information. These labels can be printed and used directly, or they can be printed on-site.
2. Medical electronic equipment labels: Medical electronic equipment labels are usually used to identify parameters such as power and voltage of the equipment, as well as to print barcodes, product models, and manufacturer information. These labels can also be printed and used directly, or printed on-site.

3. Medical product labels: Medical product labels are usually used to identify the name, specification, production date, expiration date, usage method and other information of drugs, reagents, consumables, etc. These labels can be printed and used directly, or printed on-site.
4. Circuit board labels: circuit board labels are used for circuit board management of medical electronics and medical equipment products, and are usually used to identify parameter information such as the model, manufacturer, and production date of the circuit board.
5. Wire harness label: The wire harness label is mainly used for the management of the internal wire harness of medical equipment and medical electronic products, and is usually used to identify the parameter information such as the model, manufacturer, and production date of the wire harness.
Leave us a message
Inquiry